生成准确的流式细胞术数据不仅需要经流式细胞术验证过的灵敏、特异性抗体,还取决于支持试剂的正确组合。在设计流式实验时,确定正确的试剂(例如透化缓冲液、活力染料和 Fc 阻断物)对以下非常重要:防止假阳性或假阴性并减少潜在交叉反应性,以确保您可以信赖结果。
我们联系了流式细胞术部门副总监 Christopher Manning,来回答一些常见的流式细胞术疑难排解问题。请继续阅读关于胞内和活细胞流式细胞术的细胞制备技巧,或使用以下链接跳转到感兴趣的特定主题:
活细胞对比胞内流式细胞术对比 FACS:区别是什么?
在深入探讨细胞制备技巧之前,我们先来了解一些基本定义。尽管两个术语经常互换使用,但流式细胞术和荧光激活分选 (FACS) 实际上是不同的技术,它们都用于研究复杂的细胞群体。FACS 是流式细胞术的一个子集,用于根据荧光信号分选和分离特定细胞群体。在流式细胞术提供分析数据的同时,FACS 还可以充当一种制备技术,允许物理分离目的细胞供后续实验或分析。
Manning 解释道:“值得注意的是,虽然 FACS 最常用于活细胞,但也可以对固定细胞进行分选,以用于RNA 测序等下游应用。”
活细胞流式细胞术与胞内流式细胞术有何区别?
- 活细胞流式细胞术侧重于分析活细胞上的表面标志物。这种方法通常在研究人员需要鉴定和区分某样品内部多个细胞群体(例如 PBMC)时使用,并且只需要着染细胞表面上表达的表型标志物就如此做到。在 CST 网站上,您会看到这种方法称为流式细胞术(活细胞)。
- 另一方面,胞内流式细胞术涉及分析细胞内部的蛋白质,例如磷酸化的蛋白质或转录因子,并且如果它们在实验上相关,那么也可以同时鉴定细胞表面标志物。由于抗体需要抵达胞内靶标,因此必须在分析之前首先固定并透化细胞。在 CST 网站上,您会看到这种方法称为流式细胞术(固定/透化)。
活细胞流式细胞术和胞内流式细胞术都有可能影响结果完整性的实验步骤考虑。尤其对胞内流式细胞术,实验步骤注意事项有时可能感觉令人生畏,并且采集误导性或不正确数据的可能性增加。然而,如果您考虑一些细胞制备注意事项,那么生成胞内靶标的准确流式细胞术数据可能并不像您想得那么困难。
我如何为胞内流式细胞术固定和透化细胞?
当您的靶标属胞内时,适当的固定和透化对防止假阴性和确保准确检测至关重要。固定过程使蛋白质稳定并保留它们在细胞内部的状态,而透化过程允许抗体穿透各种膜并与这些抗体的胞内靶标结合。如果细胞膜透化之前没有完全固定细胞,则胞内蛋白可能从细胞泄漏。相反,如果细胞膜或相关细胞器未充分透化,抗体将不能到达并结合于您的目的蛋白。
CST® Intracellular Flow Cytometry Kit (Methanol) #13594 包含您为保留蛋白质状态并促进抗体高效抵达胞内蛋白将所需的所有试剂。它利用无甲醇的甲醛固定细胞并利用甲醇透化。
“我们通常推荐用甲醇进行透化处理,因为其能高效穿透质膜和胞内区室,从而让抗体能到达胞内各细胞器及其他区域,”Manning 说,“在研究人员使用不太彻底的透化方法,导致抗体结合不良和错过靶标下,我们已看到一些问题。由于甲醇效果最彻底,因此我们通常推荐它作为默认的透化方法。”
相关资源:流式细胞术疑难排解指南
然而,存在甲醇与实验中抗体和/或表位不兼容的极少数情况,而使用甲醇可能导致结果不准确。不确定您的实验是否属于这个类别?CST 科学家已为您做过测试。您使用 CST 抗体时,我们会在抗体说明书或网站产品页面上推荐适当的透化实验步骤,例如使用 Intracellular Flow Cytometry Kit (Triton X-100) #51995 或 FoxP3/Transcription Factor Fixation/Permeabilization Kit #43481的那些实验步骤,如若这些方法会产生更好的结果。
试剂盒组分也可单独订购,为您设计自身实验时提供灵活性。
我如何在自己的流式细胞术样品中确认细胞活力?
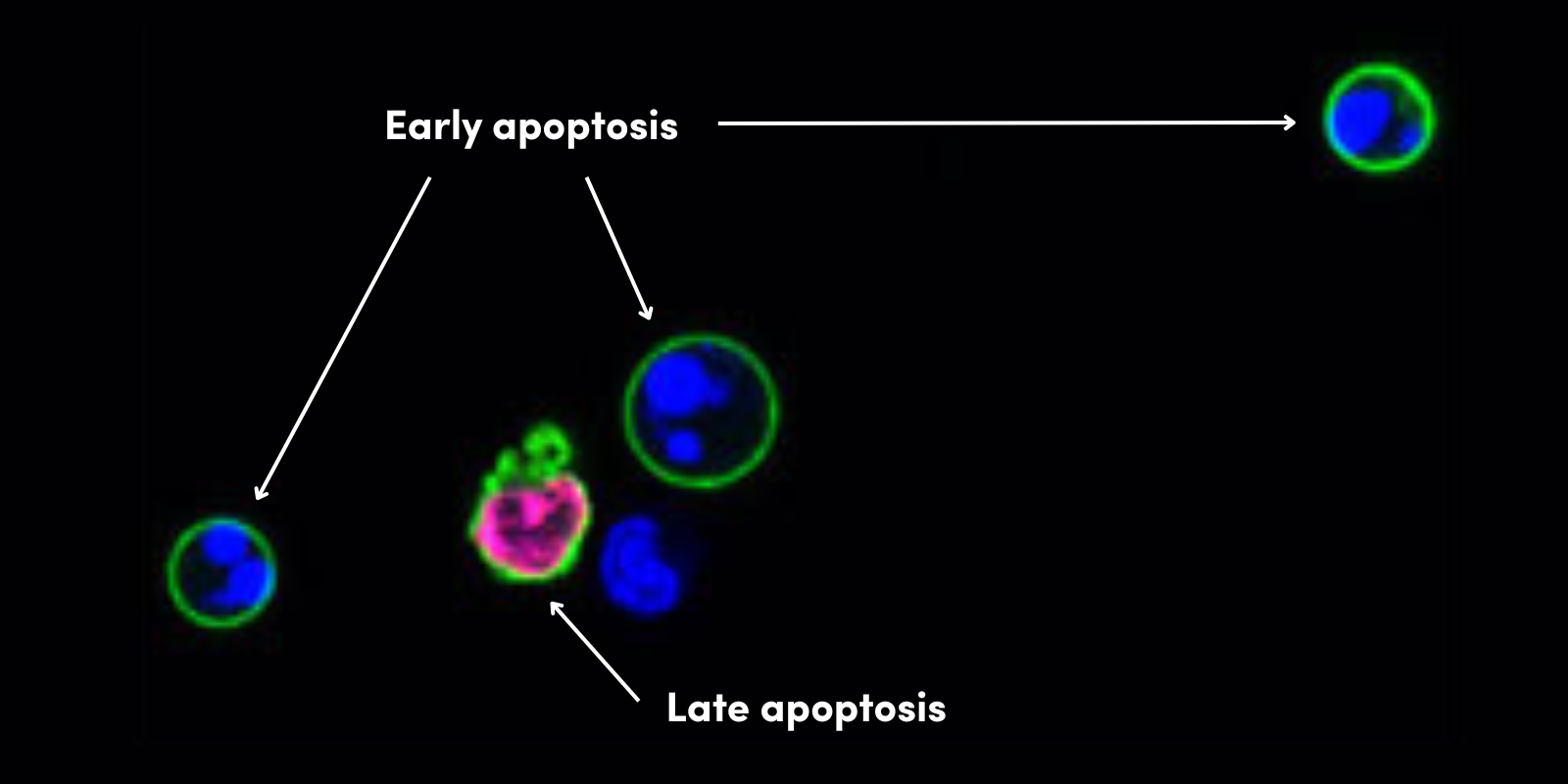
在活细胞和死细胞/垂死细胞中观察到的细胞反应、信号转导和蛋白质水平可能迥然不同。数据分析中纳入固定时样品中无活力的细胞可能使数据偏斜。可固定活力染料(例如 CST 提供的 Ghost Dyes)区分了固定样品和透化样品中的活细胞与死细胞,从而消除这种潜在的偏倚源。它们是共价结合其靶标的胺反应性染料,在固定之前明亮地标记死细胞并确保您后续可以固定并透化样品而无信号丢失。然后可以使用来自 Ghost Dye 的信号圈选无活力细胞并将它们从您的分析中排除。
“我们看到过这样的案例,研究人员使用珍贵的样品完成了整套实验,才意识到没有正确处理细胞活力,”Manning 解释道,“Ghost Dyes 等活力染料帮助您确保自身数据不因死细胞而偏斜,这让您只关注于有生物学意义的信号。”
对合并且用 Ghost Dye Blue 516 Viability Dye #99283 着染的小鼠骨髓活细胞和小鼠骨髓热灭活细胞进行流式细胞分析。指出活细胞门。
CST 提供多种颜色的 Ghost Dyes,因此可以选择在您的抗体偶联物检验组合中最契合的染料。
是否想要更多了解活力染料,包括将适用于活细胞的染料?请查看此博客:为何在流式细胞术实验中使用活力染料?
在我的流式细胞术实验中,何时应使用 Fc 阻断物?
在诸如 B 淋巴细胞、NK 细胞、巨噬细胞、中性粒细胞和肥大细胞等免疫细胞的细胞表面上的 Fc 受体可以识别所有抗体的 Fc 片段,从而导致假阳性。例如,假设您正使用兔源抗体靶向免疫细胞标志物,以表征免疫细胞和/或监测人体样品中的目的表面蛋白。无论您的靶标蛋白是什么,活细胞上的人 Fc 受体都会与您的兔抗体结合,从而导致假阳性并可能使您的结果不太明确。因此,任何时候您对具有 Fc 受体的免疫细胞进行流式实验,都应使用 Fc 阻断。
“尚不明确人 Fc 受体是否会与兔抗体结合,”Manning 解释道,“让事情进一步复杂化的是,很多时候,研究人员看到意外信号时,他们假设抗体有问题,而此时实际上,正是受体相互作用才造成假阳性。这使得 Fc 阻断至关重要——否则,您不能确定抗体是否正与正确的靶标结合,还是只黏附于受体上。”
Fc 阻断物在您向样品引入一抗之前,与细胞上的 Fc 受体结合,从而通过确保抗体不为 Fc 受体所阻隔并自由结合靶标蛋白,改进信噪比。

对 THP-1 细胞进行流式细胞分析,这些细胞用 Human Fc Receptor Blocking Solution #58948 阻断(2.5 μg/百万个细胞,10 分钟;绿色)或未阻断(蓝色)并且随后与 5 μg/mL PE 偶联同种型对照孵育 60 分钟。橙色虚线表示未阻断且未染色的细胞。阻断 Fc 受体减少了非特异性结合,如阻断的样品中荧光较低所示。
对包含髓系/单核细胞系细胞的人类活模型进行实验时,来自 CST 的 Human Fc Receptor Blocking Solution #58948 可以用来防止抗体与 Fc 受体蛋白结合,而 Mouse Fc Receptor Blocking Solution (CD16/CD32) Rat mAb #88280 可用来阻断与小鼠 Fc 受体相互作用。Fc 阻断物在活细胞实验中尤为重要,但对于胞内流式细胞术通常不需要,因为固定和透化过程破坏 Fc 受体并阻止这些受体结合抗体。
我的流式细胞术实验中应纳入哪些对照?
如果您想确保数据解读的有效性,实验对照为必备。CST 的同种型对照确认您看到的信号是特异的且不归因于非特异性结合或伪影。这些对照可以在分析固定/透化的细胞以确立非特异性荧光背景信号时使用,但在使用活细胞时尤为重要,原因在于可能出现的高水平 Fc 受体结合作用。来自同种型对照的信号确保相对于背景荧光水平,有意义地量化和圈选阳性事件。

相比 Phospho-S6 Ribosomal Protein (Ser235/236) (D57.2.2E) XP® Rabbit mAb (Alexa Fluor® 647 Conjugate) #4851 ,使用 Rabbit (DA1E) mAb IgG XP® Isotype Control (Alexa Fluor® 647 Conjugate) #2985(红色)对未处理(蓝色)或经 IFN-α(绿色)处理的 Jurkat 细胞进行流式细胞分析。
随流式术前行:使用流式细胞术应用试剂获得可信赖的数据
CST 应用科学家密切参与了开发流式实验所需的抗体和配套试剂——而且因为我们所有的流式专用试剂均由应用领域专家设计、开发和测试,故您可以相信这些试剂将在您的应用中首次有效、次次有效。从而您可以告别因样品制备不充分、对目的蛋白非特异的信号或样品中存在死细胞而导致的流式细胞术结果模棱两可。
用于流式细胞术的 CST 试剂盒和试剂
其他资源
- 要获得对照和二抗、缓冲液、检测以及其他试剂的完整列表以完成您的流式细胞术工作流程,请单击链接立即访问应用工作流程试剂手册。



 沪公网安备31011502018823号
沪公网安备31011502018823号